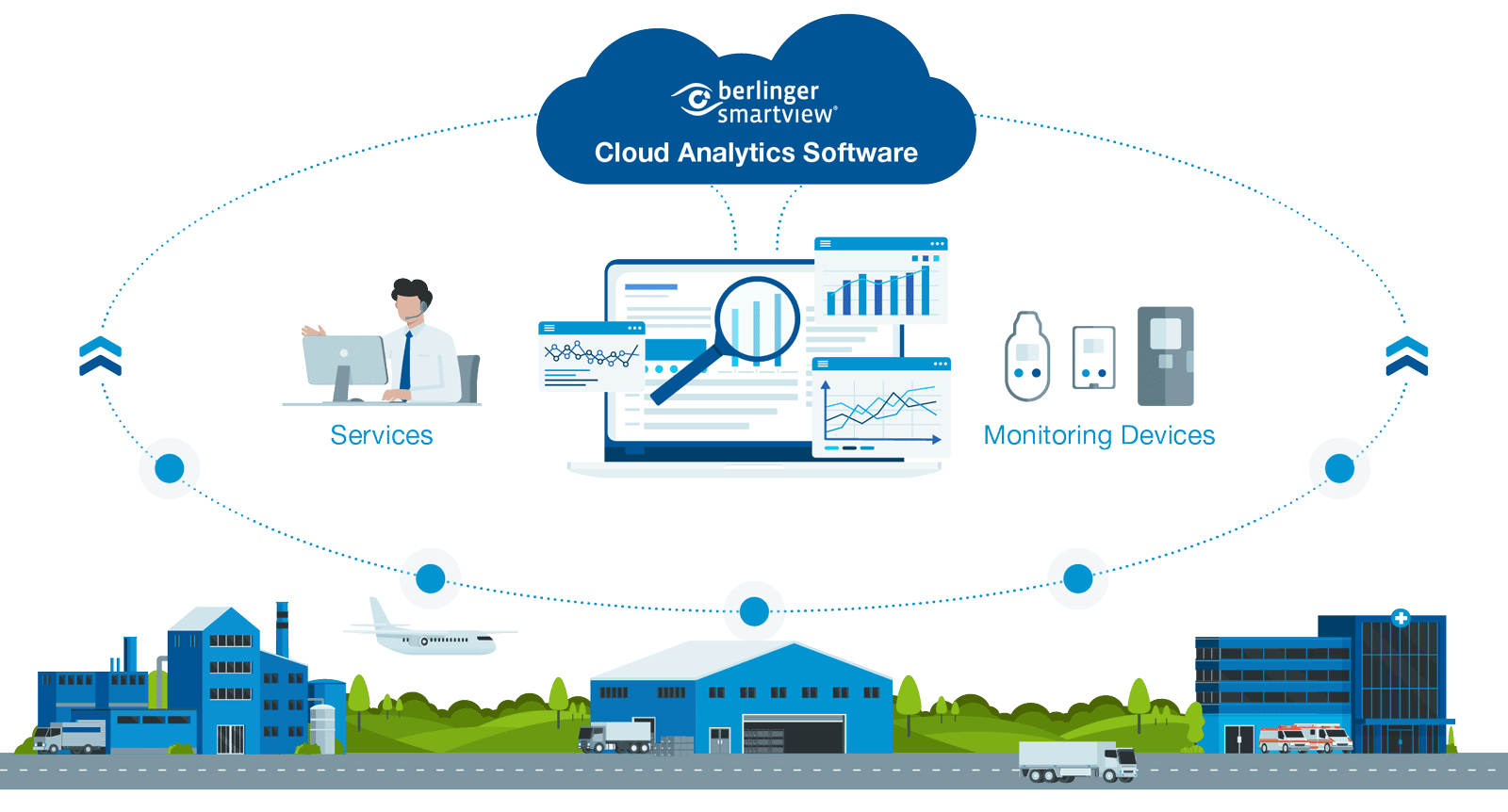
Ensuring every patient receives safe, effective treatment starts with protecting each drug kit throughout the clinical trial journey. Our automated temperature monitoring system goes beyond tracking single excursions — it monitors stability end-to-end, across the entire supply chain, so issues are detected and addressed before they become risks.
By connecting sponsors, CROs, CDMOs, and clinical sites in one streamlined platform, we help you safeguard patient safety, comply with regulations, and drive greater efficiency in the delivery of today’s most advanced therapies, from biologics to cell and gene treatments.